Our long-term goal is to understand how aberrations in molecular and cellular mechanisms governing tissue development and regeneration may lead to cancer initiation and progression. We are particularly interested in understanding the role of stem cell niches in ovarian, endometrial and prostate carcinogenesis.
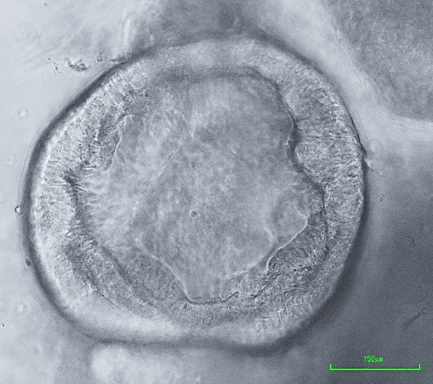
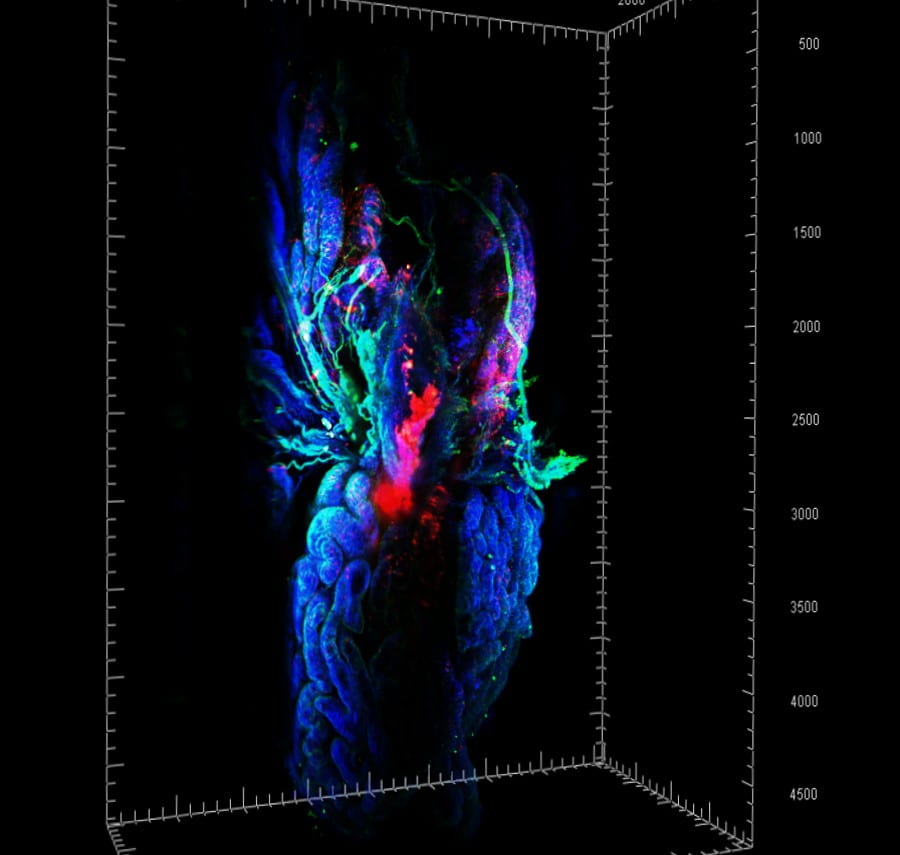
Our work is based on a combination of genetically modified mouse models, normal and neoplastic human organoids, single cell transcriptomics and 3D imaging. Successful animal models are expected to have features common with human disease, such as molecular mechanisms, course of neoplastic progression, including metastasis, competent immune system, and involvement of identical cell lineages and tissues. To address these requirements, we have prepared mouse models carrying alterations in commonly mutated in human cancers tumor suppressor genes, such as p53 (Trp53, TP53) and Rb (Rb1, RB1), Pten and miR-34 (miR-34a and miR-34b/c). Among models established by our laboratory are the first autochthonous mouse model of high-grade serous ovarian carcinoma (Flesken-Nikitin et al., 2003), metastatic prostate carcinoma (Zhou at al., 2006), luminal type B mammary carcinoma (Cheng et al., 2010), serous endometrial carcinoma (Fu et al., 2019) and squamous-columnar junction gastric cancer (Fu et al., 2020). In addition to accurate mouse models of human cancer, our current research is complemented by parallel comparative studies of human and mouse organoid systems developed in our lab (Fu et al., 2020; Rose et al., 2020).
Studies of our mouse models have allowed us fuller appreciation of the importance of cell lineage and differentiation state context in determining specific genetic and phenotypical features of resulting neoplasms. In the model of prostate cancer associated with p53 and Rb deficiency, we have shown that aggressive carcinomas arise exclusively from the prostate stem cell compartment, which is located in the proximal region of the prostatic ducts. We have also identified and characterized cancer prone stem cell niche in the ovarian surface epithelium (OSE, Flesken-Nikitin et al., 2013, Yamulla et al., 2020). Notably, OSE stem cell niche is located in the hilum region of the mouse ovary, the transitional/junction area between OSE, mesothelium and oviductal epithelium. Our recent findings show that susceptibility of other transitional zones to malignant transformation may also be explained by presence of stem cell niches (Schmoeckel et al., 2017, Fu et al., 2020).
One of the main limiting factors in the elucidation of cancer pathogenesis is our insufficient understanding of cell hierarchy of target organs, including such essential aspects as stem cell identity, cell fate decision and homeostasis. Addressing this challenge is the focus of our current research (Fu et al., 2019). We are also interested in identification and characterization of molecular and cellular mechanisms responsible for preferential susceptibility of stem cells to malignant transformation, such as p53/mir-34/MET pathway (Hwang et al., 2011, Cheng et al, 2014), osteopontin-CD44 pathway (Fu et all, 2020), and neuroendocrine signaling (Cheng et al., 2020).